
COVID-19 rapid test kits in high demand as infections skyrocket
Latest
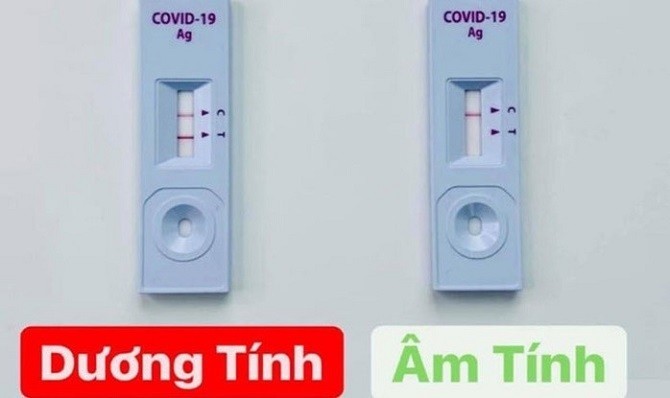 |
| The test kits are diverse and have different origins. |
Local residents are rushing to stock up on these products as the daily number of infections in Hanoi has increasing considerably, reaching more than 5,000 these days. This has led to an increase in purchases of rapid test kits in major pharmacy streets such as Chau Long, Quang Trung, Pho Hue, Le Van Huu, Cau Giay, and Thanh Nhan.
The test kits are diverse and have different origins, including those produced in China, Turkey, and the Republic of Korea. Their prices also vary, depending on their origin and manufacturer, with test kits changing hands at VND52,000 to VND110,000 each on average.
Nguyen Ngoc Anh, a resident living on Kim Lien street in Dong Da district, said has bought rapid test kits to test her family members before they go to their schools or offices as the infection risk in the community is rather high.
Similarly, many other local people are rushing to pharmacies to purchase such kits to ensure the kits are available at home for testing at any time. High demand has also caused kit prices to rise by VND15,000 each.
Chu Xuan Kien, director of the Hanoi Market Management Department, pointed out that besides pharmacies, many individuals are also trading these items through social media, creating difficulty for the inspection and handling of violations.
If any trading of test kits which are not licensed by the Ministry of Health or have unknown origin and unreasonable prices is detected, then the General Department of Market Management will co-ordinate efforts with competent agencies to promptly handle them, he noted.
Apart from rapid test kits, herbal products used for inhalation such as lemongrass, ginger, and lemon to prevent COVID-19 pandemic are also much sought after these days. Their prices have risen by up to three folds due to the increasing demand and limited supply.
The Drug Administration of Viet Nam (DAV) under the Ministry of Health on February 17 issued a list of three COVID-19 treatment drugs containing the active ingredient Molnupiravir, which has now been granted certificates of registration for conditional circulation.
The three drugs are Molravir 400 produced by Boston Vietnam Pharma; Movinavir 200 mg manufactured by Mekophar Chemical Pharmaceutical; and Molnupiravir Stella 400 produced by Stellapharm J.V Co., Ltd.
Molnupiravir, the antiviral drug, has been approved for treating COVID-19 patients with mild to moderate symptoms. Studies indicate that the medication can help reduce the numbers of hospitalisations and deaths in COVID-19 patients.
The approval of these drugs with conditional circulation is therefore expected to control rapid test kit and medicine markets.













